We are fascinated by host-parasite interactions. We study the host immune response to parasites and how parasites evade and/or suppress immunity. We are studying proteins that are released by parasitic nematodes during infection and are working to identify how they interact with host biology. We work with nematode parasites of insects and mammals using a variety of insect hosts. We study host immunity using bacterial pathogens, nematode parasites, and other immune insults.
 Figure 1. The life cycle of entomopathogenic nematodes. 1) The IJ stage is a developmentally arrested third larval stage and is the only free-living stage; all other stages exist within the host. These free-living IJs find a host, enter usually through natural openings, and initiate parasitism inside the hemolymph. 2) EPN IJs release their bacterial symbiont into the host hemolymph and together, both the bacteria and the IJs release toxic molecules into the host to kill it. 3) The nematodes develop and feed on their growing bacterial symbiont and liquified insect tissues. 4) Nematodes reproduce, potentially going through 2-3 generations, depending on the size of the host and number of nematodes that initiate the infection. 5) When the population density is high and resources begin to deplete, new IJs develop and disperse, carrying the symbiotic bacteria to new hosts. Figure from (Baiocchi et al. 2017, Scientific Reports).
Figure 1. The life cycle of entomopathogenic nematodes. 1) The IJ stage is a developmentally arrested third larval stage and is the only free-living stage; all other stages exist within the host. These free-living IJs find a host, enter usually through natural openings, and initiate parasitism inside the hemolymph. 2) EPN IJs release their bacterial symbiont into the host hemolymph and together, both the bacteria and the IJs release toxic molecules into the host to kill it. 3) The nematodes develop and feed on their growing bacterial symbiont and liquified insect tissues. 4) Nematodes reproduce, potentially going through 2-3 generations, depending on the size of the host and number of nematodes that initiate the infection. 5) When the population density is high and resources begin to deplete, new IJs develop and disperse, carrying the symbiotic bacteria to new hosts. Figure from (Baiocchi et al. 2017, Scientific Reports).
Parasitic nematodes are often asymptomatic and cause little pathology to their host. We are interested in understanding how nematode parasites evade and/or suppress the host immune response. Currently we are investigating the protein secretions of a particular guild of insect-parasitic nematodes called entomopathogenic nematodes (EPNs) (Figure 1). We are exploring the effects of these nematode proteins on host immunity (Figure 2). We have found that Steinernema carpocapsae and S. feltiae infective juveniles release hundreds of proteins when they initiate active parasitism, and that these proteins are toxic to a variety of insect hosts (Lu et al. 2017, PLoS Pathogens; Chang et al. 2019, PLoS Pathogens; Okakpu & Dillman, 2022, Journal of Parasitology). Now that we have identified hundreds of proteins released by parasitic nematodes, we are interested in characterizing these proteins and identifying which of the proteins in the nematode arsenal cause damage to the host and which are involved in modulating host immunity. This work is largely biochemical and relies on our ability to produce recombinant versions of these proteins to work with. So far we have worked with fatty acid- and retinol-binding proteins (FARs) (Parks et al., 2021, PLoS Pathogens; Parks et al., 2022, PLoS Pathogens), ShK-domain-containing proteins (Lima et al., 2022, Pathogens), and secreted phospholipase A2 enzymes (Parks et al., 2023, Frontiers is Immunology). We are primarily using Drosophila melanogaster as a model host. This allows us to produce transgenic flies that express nematode effectors and to leverage the powerful genetics of the fly to study these interactions.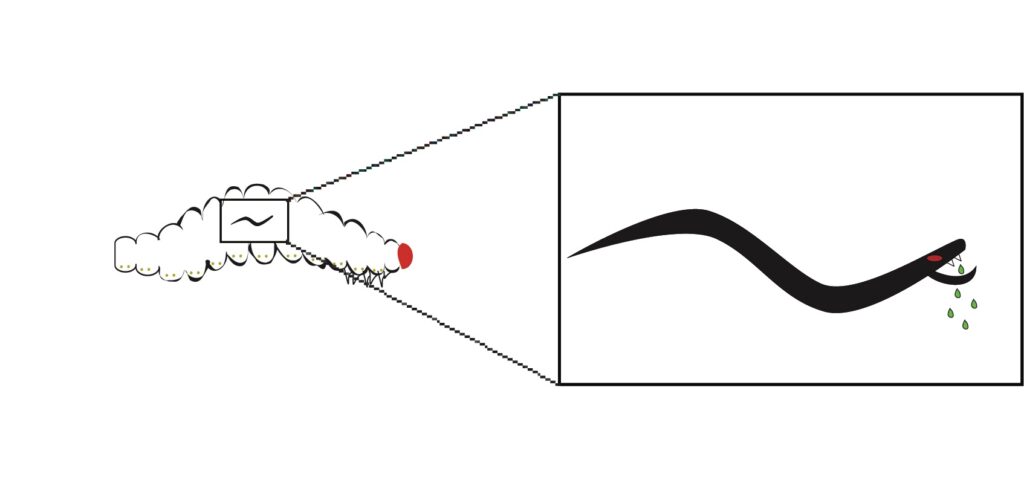
Figure 2. Activated infective juvenile nematodes release proteins and small molecules into the host. While hundreds of these proteins have been identified in many different species of nematodes, few have been studied in mechanistic detail. We are interested in performing functional analyses of these effector proteins.
We have some videos of the nematodes we work with and some of the assays we use up on our youtube site: https://www.youtube.com/user/Wormherder

